When it comes to the world of chemistry, one of the most fundamental concepts to grasp is the understanding of acids, bases, and pH levels. These concepts are crucial not only for chemistry enthusiasts but also for anyone working in the tech industry where chemical reactions play a significant role. In this article, we will delve deeper into what acids and bases are, how pH levels are determined, and why it is essential to have a basic understanding of these concepts.
Acids: The Proton Donors
Acids are chemical compounds that are known for their ability to donate protons in a chemical reaction. This means that acids have a high concentration of hydrogen ions (H+). When an acid reacts with water, it releases hydrogen ions which then react with other substances, causing chemical reactions to occur. Some common examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and acetic acid (CH3COOH).
Bases: The Proton Acceptors
On the other hand, bases are chemical compounds that are known for their ability to accept protons in a chemical reaction. Bases have a high concentration of hydroxide ions (OH-) which can combine with hydrogen ions to form water. This reaction is what gives bases their characteristic slippery feel. Some common examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
pH Levels: The Measure of Acidity or Alkalinity
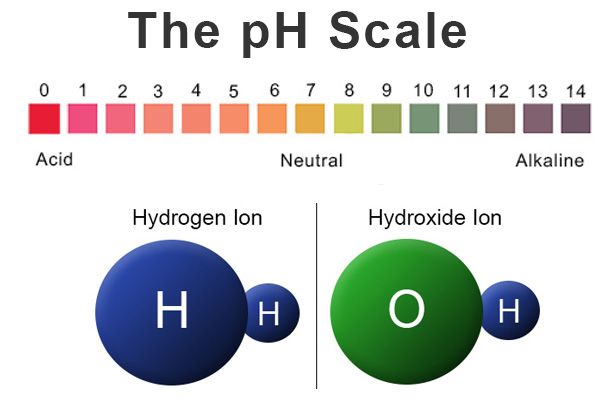
pH levels are a measure of the acidity or alkalinity of a substance. The pH scale ranges from 0 to 14, with 7 being considered neutral. Substances with a pH value below 7 are considered acidic, while substances with a pH value above 7 are considered alkaline (basic). The lower the pH value, the more acidic a substance is, and the higher the pH value, the more alkaline a substance is.
The pH level of a substance is determined by the concentration of hydrogen ions present in the solution. Acids have a higher concentration of hydrogen ions, resulting in a lower pH value. Bases, on the other hand, have a lower concentration of hydrogen ions and a higher concentration of hydroxide ions, leading to a higher pH value.
Importance of Understanding Acids, Bases, and pH Levels in Tech
Having a basic understanding of acids, bases, and pH levels is crucial in the tech industry for several reasons. Many processes in technology rely on chemical reactions that involve acids and bases. For example, in the manufacturing of electronic devices, acids and bases are used in etching processes to create circuit boards. Understanding how acids and bases interact can help prevent accidents and ensure the safety of workers.
Additionally, in fields such as data analysis and data storage, pH levels play a role in maintaining the integrity of equipment. For example, in data centers, maintaining the pH levels of cooling systems is essential to prevent corrosion and extend the life of equipment. Understanding how pH levels can affect equipment can help tech professionals make informed decisions and prevent costly repairs.
Overall, a solid understanding of acids, bases, and pH levels is essential for anyone working in the tech industry. By understanding how these chemical concepts work, tech professionals can make informed decisions, prevent accidents, and ensure the efficiency and longevity of equipment.
In conclusion, acids, bases, and pH levels are fundamental concepts in chemistry that play a critical role in various industries, including the tech industry. By understanding the properties of acids and bases and how pH levels are determined, tech professionals can enhance their knowledge and make informed decisions in their work. So, next time you encounter a chemical reaction in your tech work, remember the basics of acids, bases, and pH levels to ensure safety and efficiency.